Submit travel and reimbursement requests anytime, anywhere.
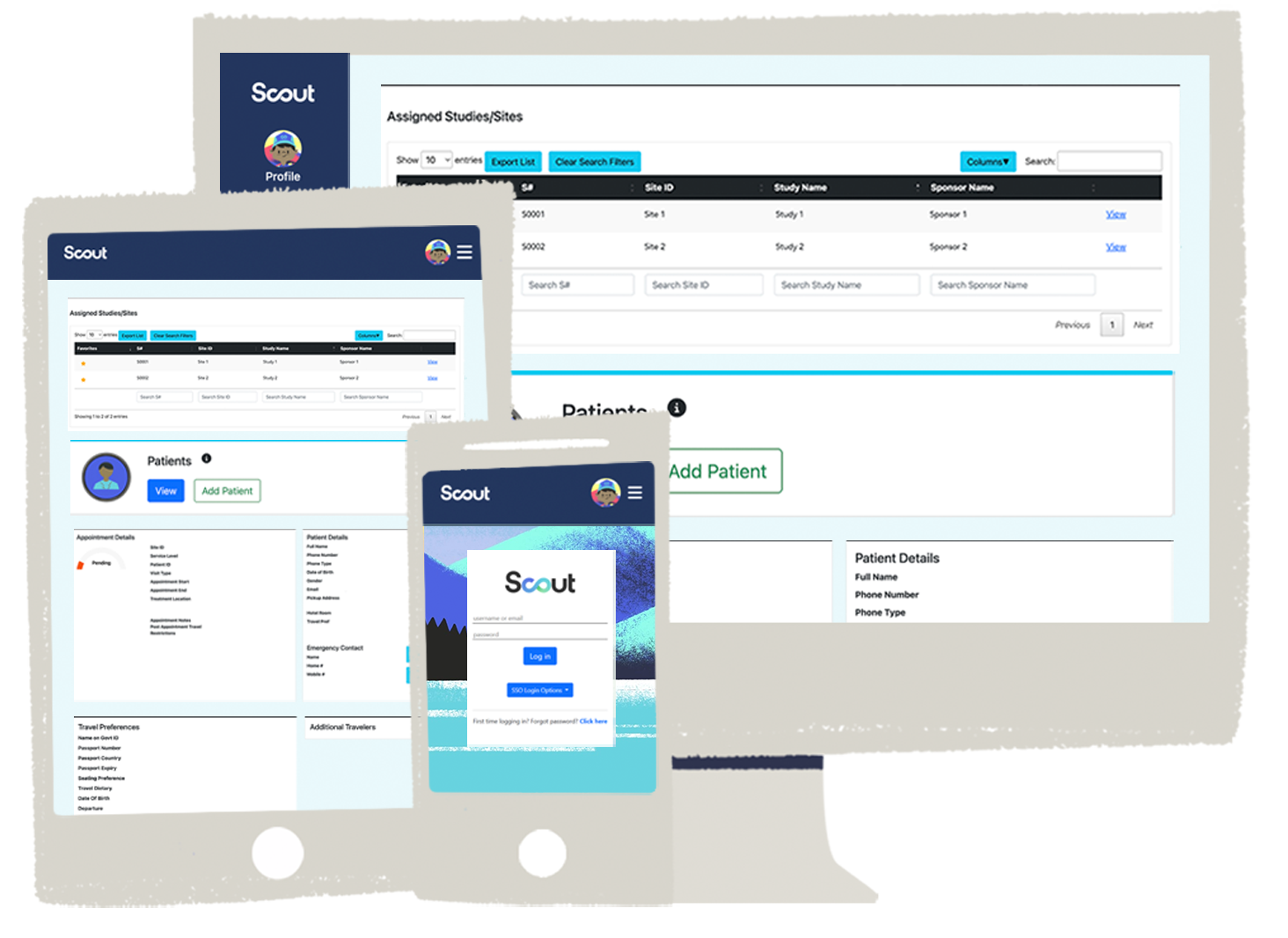
Scout Portal
Everything you need to manage patient payments, travel, and logistics is right at your fingertips. The Scout Portal serves as a secure, user-friendly patient portal for clinical trials, allowing participants and site staff to submit and track requests anytime, anywhere—from desktop to mobile and every screen in between.
Designed for users like you
Scout is available through multiple single sign-on (SSO) integrations, giving you seamless access from the systems you already use.
Fewer logins. Less juggling. More time to focus on what matters.
Smart features built for clinical research
Submit requests from wherever you are—out on the trail, in the clinic, or even from your car.
Every submission is securely processed through the portal, and our team keeps you updated at each step.
Fast, compliant, and designed to make participation easier for everyone involved.

Always compliant, always secure
Built to meet HIPAA, GDPR, MR-001, and other country-specific privacy standards, the Scout Portal keeps participant data safe and confidential.
All activity is encrypted and logged to support audit readiness and regulatory compliance across studies worldwide.
Accessibility at its core
As a patient portal for clinical trials, the Scout Portal supports over 200 languages and works seamlessly on any device.
Its inclusive design ensures that every participant, regardless of location or technology access, can use the portal easily and independently.
No-hassle expense reimbursement
Tired of chasing receipts? Submit reimbursements for meals, rideshares, or parking in seconds through the Scout Portal.
- Transparent tracking throughout the process
- Flexible payment options, including ScoutPass® debit card, PayPal, and direct bank transfer
Participants see exactly where things stand, while sites and sponsors save time on administrative back-and-forth.
Travel management made easy
Managing clinical trial travel is effortless with the Scout Portal.
From booking transportation to arranging hotel stays near trial sites, every detail is handled smoothly and securely.
- Ground, air, and rail booking tools are built in
- Accessible accommodations are available for participants with unique needs
Reporting and visibility built in
Experience one centralized workspace for tracking reimbursement and travel activity across clinical studies.
Sponsors, CROs, and sites can view real-time request status, access consolidated reports, and confirm that every transaction is processed correctly and on time.
It’s transparent, audit-ready, and designed to support compliance without extra work.

Download the Scout App
It’s the Scout Portal, right on your mobile device. Submit and track requests from anywhere! Tablet? Smartphone? It’s all good, and good-to-go with the Scout App.
If you’re already set up in the portal, download the app today in:

Register. Submit. Go!
The Scout Portal combines simplicity, security, and speed in one easy-to-use package. Accessible, efficient, and built for everyone involved.
Want to see the portal in action? Contact us today—we’re here to support your study’s success every step of the way.
.png?width=1387&height=800&name=Scout_Logo_RGB%20(1).png)